Introduction:
Recent evidence has emerged to support the use of pediatric-inspired regimen as a standard of care in Adolescents and Young Adults (AYA) patients diagnosed with Philadelphia-negative acute lymphoblastic leukemia (Ph- ALL). The backbone of the pediatric regimen is high cumulative doses of steroids, asparaginase, vincristine and more intensive CNS prophylaxis. Most literature, however, has been in the western population, with few data reported in Asia. MASPORE protocol is a locally designed pediatric-inspired regimen for treatment of ALL in AYA. The protocol uses a 3- or 4-drugs induction regimen (L-asparaginase, Vincristine, Dexamethasone +/- Daunorubicin) based on standard or high-risk disease. PCR-based measurable residual disease (MRD) marker was utilized for disease monitoring and dynamic risk stratification, which led to subsequent intensification of regimen as needed.
Methods:
In this IRB approved retrospective analysis, patients aged 18-39 years old with Ph- ALL who received MASPORE regimen from January 2009 to December 2021 at the National University Hospital Singapore were identified via our institution database. Treatment outcomes, including measurable residual disease (MRD) monitoring, and toxicity profile were analyzed. Survival outcomes were estimated using Kaplan-Meier method. High risk disease at diagnosis was defined as high white blood cell (>30x10 9/L for B-ALL, >100x10 9/L for T-ALL), hypodiploidy, t(4;11) and other MLL gene rearrangement.
Results:
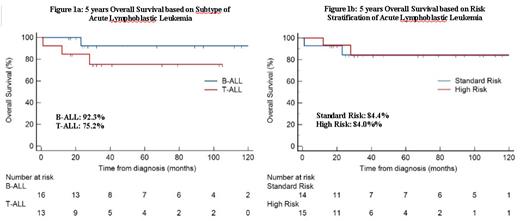
32 patients were identified during the study period; 3 patients were excluded due to incomplete data. The median age was 23 years old (range 18-33 years) with majority being male (86.2%). 16 patients (55.2%) had B-ALL and 13 (44.8%) had T-ALL. High risk disease was present in 7 (43.8%) B-ALL patients and 8 (61.5%) in T-ALL patients. 2 patients in B-ALL group had CNS involvement at diagnosis. The majority (96.5%) achieved complete remission after the induction phase. 1 patient died within first 1 month of treatment due to pancreatitis. 5 patients in high risk group received allogenic stem cell transplantation (alloSCT), whereas 2 patients with standard risk required alloSCT due to relapse and persistent MRD positivity. MRD result was available for 26 patients. In the entire cohort, MRD negativity was achieved in 22 patients (75.9%). Subtype was not associated with achievement of MRD negativity (80% in B-ALL vs 90.9% in T-ALL). High risk patients however had a lower odds of achieving MRD negativity after induction (OR 0.114 95% CI 0.017-0.742, p=0.021). The median follow-up for survivors was 41 months (range 27 to 89 months). Relapse and mortality rate was low at 13.8% each. 5-year OS for patients with B-ALL and T-ALL were 92.3% and 75.2%, respectively (Figure 1a). 5-year OS for patients with standard and high-risk disease were 84.4% and 84.0%, respectively (Figure 1b). Delayed achievement of MRD negativity (i.e. after induction) was not associated with inferior survival outcomes, albeit our sample size was small. In terms of adverse events, Asparaginase-related toxicities included 11 patients (37.9%) with thrombotic events (4 cerebral venous thrombosis and 7 deep vein thrombosis), and 5 patients (17.2%) with grade 2 or more pancreatitis. 7 patients (24.1%) had at least 1 episode of grade 3 or 4 transaminitis. 7 patients (24.1%) had grade 3 or 4 sepsis during the course of treatment. 2 patients developed osteonecrosis of the hip requiring operation and cessation of steroids.
Conclusion:
Our locally designed MASPORE protocol tailored in accordance to risk stratification demonstrated promising survival outcomes. Similar outcomes observed in standard and high-risk disease suggests the benefit of such an approach. Toxicities were mainly related to L-asparaginase use. Individualized dosing based on drug level monitoring will help to further optimize the treatment intensity with reduced toxicities.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal